An electric battery is a device consisting of one or more electrochemical cells that convert stored chemical energy into electrical energy. Each cell contains a positive terminal, or cathode, and a negative terminal, or anode. Electrolytes allow ions to move between the electrodes and terminals, which allow current to flow out of the battery to work.
If you introduce zinc foil in a solution of copper sulphate the solution becomes more clear and the foil becomes more black, this is a redox reaction because the copper is reduced and it is deposited on the zinc that is oxidized and makes ions Zn++.
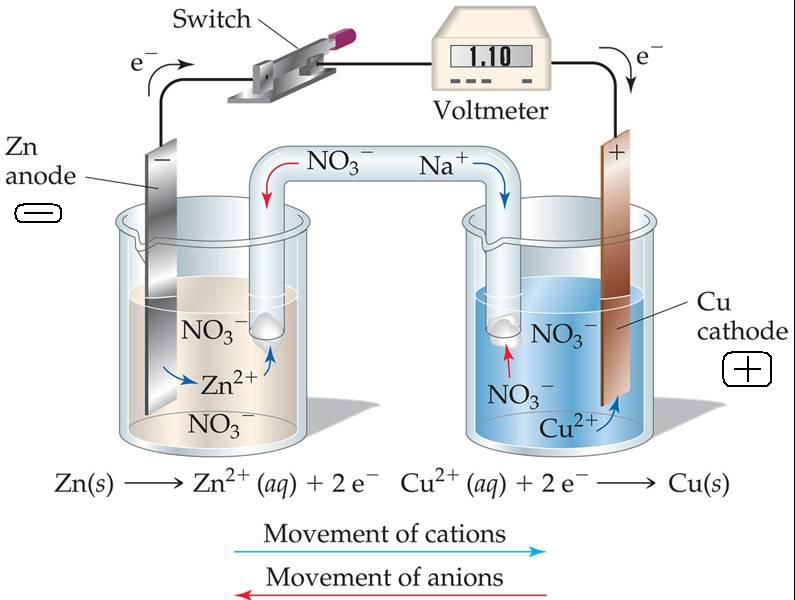
This chemistry phenome to make energy is used by the daniell battery:
When you put zinc bar in a sulphate of zinc, some atoms of zinc are detached from the bar and produce electrons extern of the metal.
When you put copper bar in a sulphate of copper some positive ions of the solution are deposited on metal.
If you connect this two systems one with the other the electrons made of the zinc bar are moved to the copper bar and this produces electric energy.
| oxidation | Zn —-> Zn2+ + 2e |
| reduction | Cu2+ + 2e —-> Cu |
| ——————————— | |
| Zn + Cu2+—-> Zn2+ + Cu |
The zinc bar is the anode (negative pole) and the copper bar is the cathode (positive pole). To make both the solutions neutral (without excess of positive or negative ions) it is necessary to connect each the solutions with a “salt bridge” made by KCL.
At the end of this process the zinc is oxidized and probable it’s dissolved and the copper is reduced.
If this chemical process is reversible you can recharge the battery:
During charging, the positive active material is oxidized, producing electrons, and the negative material is reduced, consuming electrons. These electrons constitute the current flow in the external circuit. The electrolyte may serve as a simple buffer for internal ion flow between the electrodes, as in lithium-ion and nickel-cadmium cells, or it may be an active participant in the electrochemical reaction, as in lead acid cells.